 Air Pollution:
Air Pollution:Air is polluted due to natural and man made pollutants. Gases such as CO2,SO2,CO,NO,SO3,NO2,H2S etc.
are continuously released into the atmosphere through natural activities as well as human activities. Thus contaminated
of air with harmful gases,dust,smoke,etc. is known as air pollution. Air pollutants disturb the dynamic equilibrium in
the atmosphere there by effect on man and his environments.
Causes of Air Pollution: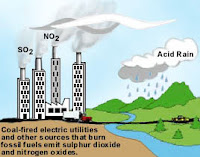
1) Burning of the fossil fuel viz petrol, coal, diesel, etc.
2) Smoke from vehicles, industries.
3) Lunching of rocket.
4) Volcanic eruption.
5) Bombarding.
6) Respiration and decay of animals and plants.
Effect of Air Pollution:
1) Man suffers from lungs and heart diseases.
2) Causes the sterility of human as well as animals and birds.
3) Increase the green house effect.
4) Effect on dynamic equilibrium of Eco-system.
5) Increase in temperature of earth.
6) Causes seasonal change.
Control of Air Pollution:
1) By afforestation.
2) By using renewable source of energy.
3) By adding TEL in petrol.
4) By keeping vehicles properly tuned for the autoignition of fuel.
5) By using tall chimney in factories and houses.
Photochemical Smog:
The word photochemical smog is formed by the combination of smoke and fog. It occurs in big cities with warm,
dry, sunny climate. The primary pollutants like NO, NO2, CO, hydrocarbon undergo in the formation of photochemical smog.
Photochemical smog is characterized by brouhaha fumes which irritates eyes and lungs beats leading to the cracking of
rubber and extensive damage of plant life. The photochemical smog is formed when pollutants are trapped in stagnant air
and intense of sunlight is present primary pollutants undergo a number photochemical reaction in presence of intense
of sunlight to give oxide of nitrogen which combine with unburnt hydrocarbons leads to formation of aldehyde, Ketone,
peroxyacyl nitrate as photochemical smog.
Photochemical smog has several effect as:
1) It irritates eyes, throat, nose, lungs.
2) It reduces visibility and affect the air and road traffic.
3) It causes fading of dyes, paints, damage cotton.
Green house effect and global warmingHeating up of the earth's atmosphere due to the trapping of infrared radiation longer wavelength by CO2 in the atmosphere
is called the green house effect. Our earth is covered by the blanket of several gases released by human activities and
natural process. These gases from thick layers expanded from earth through which considerable portion of solar radiation
pass up to the earth surface. These radiation are absorbed and reflected back again in the form of infrared and heatwave.
This heatwave transmitted to the several layers of gases and causes the earth surface warming. This phenomenon is known
as Green house effect.
The gases responsible for green house effect are CO2, CFO, N2O, water vapor. The relatives contribution
of the radiatively active gases to increase temperature are shown in figure.
Effect of Green house effect as global warming
1) It causes the crops cultivation, growth of plants, monsoon rains.
2) Excessive melting of polar ice and causes flooding and submerging of many low-lying areas.
3) Effect on breeding of birds and fishes.
4) Increase in incidents of infectious diseases.(malaria, dengue, etc.)
5) Reduction in several production.
Control of Green house effect1) By reducing consumption of fossil fuels drastically.
2) By increasing efficiency of I.C engine.
3) By uses of bio-gas.
4) By banning of use of CFCs.
5) By afforestation.
Ozone hole (depletion of ozone layer and CFCS)
Ozone is formed in the upper atmosphere as oxygen absorb the high energy UV rays of sun.
Its concentration in the stratosphere is about 10ppm where as it is only about 0.04ppm near the earth surface. The Ozone
sphere acts as an absorbent of all harmful radiation coming from the sun and space and hence behave as the protective
umbrella for the living organism on the earth. However, NASA scientists in 1958 discovered a hole in the first time in
the ozone layer of the stratosphere over Antarctica. It is mainly due to chloro-flouro carbon. These CFC's are excessively
used by the human beings as coolants in refrigerators, air conditioners etc. When these CFC's comes to the earth atmosphere
absorb UV light and decompose to give chlorine atoms which destroy Ozone molecules in stratosphere as follows:
MechanismThus once the chlorine atom is produced from decomposition of CFC, hundred of thousands of Ozone molecules are destroyed
through above same chain reaction. It is estimated that one molecule of CFC can destroy 100,000 molecules of Ozone.
Besides these CFC's other factors responsible for the depletion of ozone layer are:
1) Nuclear test
2) Combustion of fossils fuel
3) Different pollutants like NO, CO, CH4 etc.
4) Excessive use of nitrogenous fertilizers.
5) Supersonic trans port, rocket lunching etc.
Adverse effect of Ozone layer depletion
1) The harmful UV rays can reach the earth surface directly and causes skin cancer, blood cancer.
2) UV-radiation causes the reduction in crops productivity.
3) It also causes eye cancer.
4) It brings rust able changes in global warming and in the climate.
Protection of Ozone layer1) By discouraging the use of CFCs
2) By providing suitable procedure to recapture the released CFCs from coolants.
3) The use of plastics foam must be boy coated.
4) By lunching worldwide awareness prognoses regarding the protection of ozone layer.
Dissolved Oxygen(DO) It is an important water quality parameter and define as the oxygen dissolve in water which is consumed by the oxidation of organic matter. The optimum value for good water quality is 4-6mg/liter of DO which consumes healthy aquatic life in a water body. However DO values indicates water pollution.
Estimation of DO Dissolve oxygen levels depends upon the physical, chemical and biological activities of water body. The analysis of DO plays vital role water pollution control activities. It can be estimated by iodometric titration method.
Iodometric method DO is allowed to react with I- from I2 which is then titrated with standard solution of Na2S2O3. Fast quantitative reaction occurs by the addition of man(II) salts in strongly alkaline medhod.
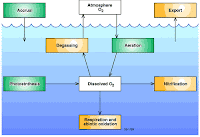 Bio chemical oxygen demand (BOD)
Bio chemical oxygen demand (BOD) It is defined as the quantitative measure of the oxygen used by the organic matter in a sample of polluted
water during a five days incubation period in the dark at 25 degree Celsius. It is evaluated experimentally by determining concentration of dissolved O2 in the beginning and at the end of a five day period in a seated water. The more the value of BOD more is polluted water.
Determination of BOD
An empirical semi quantitative method base on oxidation of organic matter by suitable microorganism
during five days period is applied to determine BOD. This parameter is commonly measured by the quantity of CO2 utilized by
suitable aquatic micro organism during five days period.
The selection of microorganism(seed) is very important and the result is obviously not reproducible. In domestic waste water or surface water, such organism are already present and seeding is unnecessary but when sample is deficient of organism feeding is necessary.
Chemical oxygen Demand(COD) This is an index which measure the effect of pollution on dissolved oxygen. It notifies the organic content of water (oxygen demanding substance in water). In COD test, an oxidant instead of O2 is used to degrade the pollutant in the sample. The amount of added oxidant consumed is experimentally measured to calculate the equivalent oxygen required by waste materials for degradation of pollutants.
Determination of COD Acidified Cr2O7s commonly used for such COD test. The COD includes some pollutants (eg:cellulose) which are not biodegraded bu oxygen, as a result of which the COD value for a water sample is higher than BOD value.
In the Cod test, the basic equation involved are
Therefore, 1 mole of C2r2O7 consumed=6/4=1.5 moles of oxygen used.
When a water sample is highly polluted with organic wastes,

the oxygen demand will exceed the maximum equilibrium solubility of O2 in water, Such a water sample may not have any dissolved O2.

 Such interactions between nearby galaxies, which may ultimately results in galaxy merging, may induce episodes of significantly increased star formation, producing what is called a star-burst galaxy. Small galaxy that lacks a coherent structure could also be referred to as irregular galaxies.
Such interactions between nearby galaxies, which may ultimately results in galaxy merging, may induce episodes of significantly increased star formation, producing what is called a star-burst galaxy. Small galaxy that lacks a coherent structure could also be referred to as irregular galaxies.
 surround immense voids in the universe. Although it is not well understood, dark matter appears to account for around 90% of the mass of most galaxies. observational data suggests super-massive black holes may exist at the center of many, if not all, galaxies. They are proposed to be the primary cause of active galactic nuclei found at the core of some galaxies. The milky way galaxy appears to harbor at least one such objects within its nucleus.
surround immense voids in the universe. Although it is not well understood, dark matter appears to account for around 90% of the mass of most galaxies. observational data suggests super-massive black holes may exist at the center of many, if not all, galaxies. They are proposed to be the primary cause of active galactic nuclei found at the core of some galaxies. The milky way galaxy appears to harbor at least one such objects within its nucleus.
 the faint band of light known as the milky way. In the astronomical literature, the capitalized word "Galaxy" is used to refer to our galaxy, the milky way, to distinguish it from the billions of other galaxies. The term milky way first appeared in the English language in a poem by Chaucer.
the faint band of light known as the milky way. In the astronomical literature, the capitalized word "Galaxy" is used to refer to our galaxy, the milky way, to distinguish it from the billions of other galaxies. The term milky way first appeared in the English language in a poem by Chaucer.
 atmosphere, in the region of the world which is continuous with the heavenly motions." The Arabian astronomer Alhazen (965-1037A.d.), refuted by making the first attempt at observing and measuring the Milky way`s parallax, and thus "determined that because the Milky way had no parallax, it was very remote from the earth and did not belong to the atmosphere. "
atmosphere, in the region of the world which is continuous with the heavenly motions." The Arabian astronomer Alhazen (965-1037A.d.), refuted by making the first attempt at observing and measuring the Milky way`s parallax, and thus "determined that because the Milky way had no parallax, it was very remote from the earth and did not belong to the atmosphere. "















